DISCLAIMER:
ALETTA 1.0 is using the validated questionnaire EQ-5D by EuroQol Research foundation. It is not a medical device, and has no medical applications.
What is ALETTA 1.0?
ALETTA 1.0 is a cloud base Real-World Data management platform built in with the validate questionnaire EQ-5D, a standardised instrument for generic health status. ALETTA is designed for self-completion and as such captures information directly from the patient using a mobile App available on the Android and Apple Store.
Use ALETTA 1.0 to assess Patient Quality of Life under MYCB1 cannabinoids treatment:
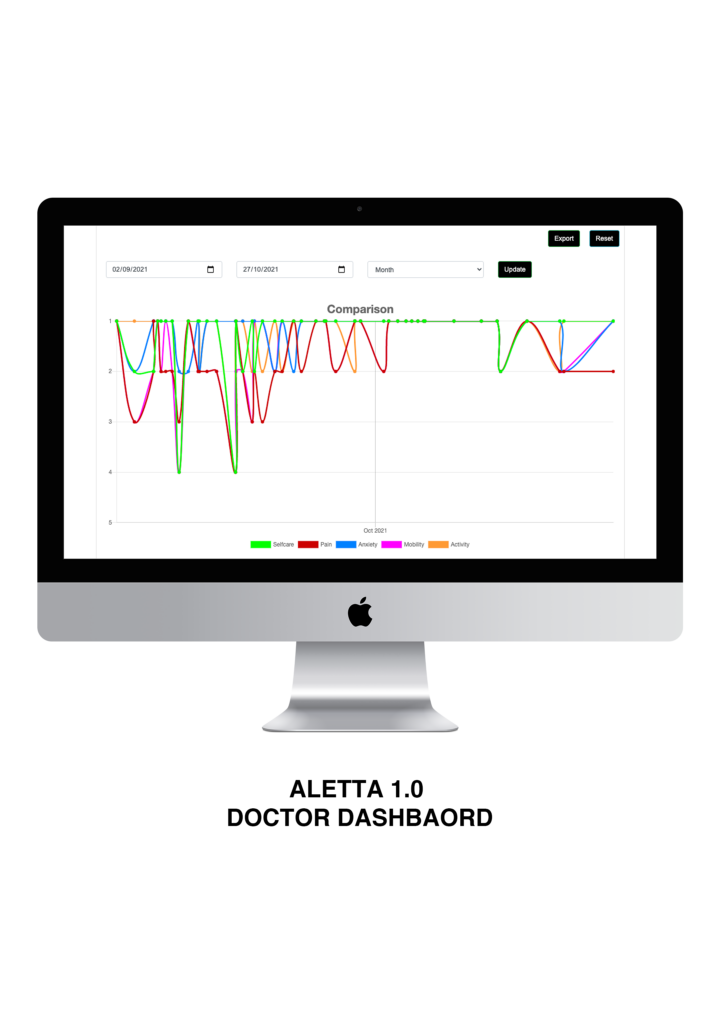

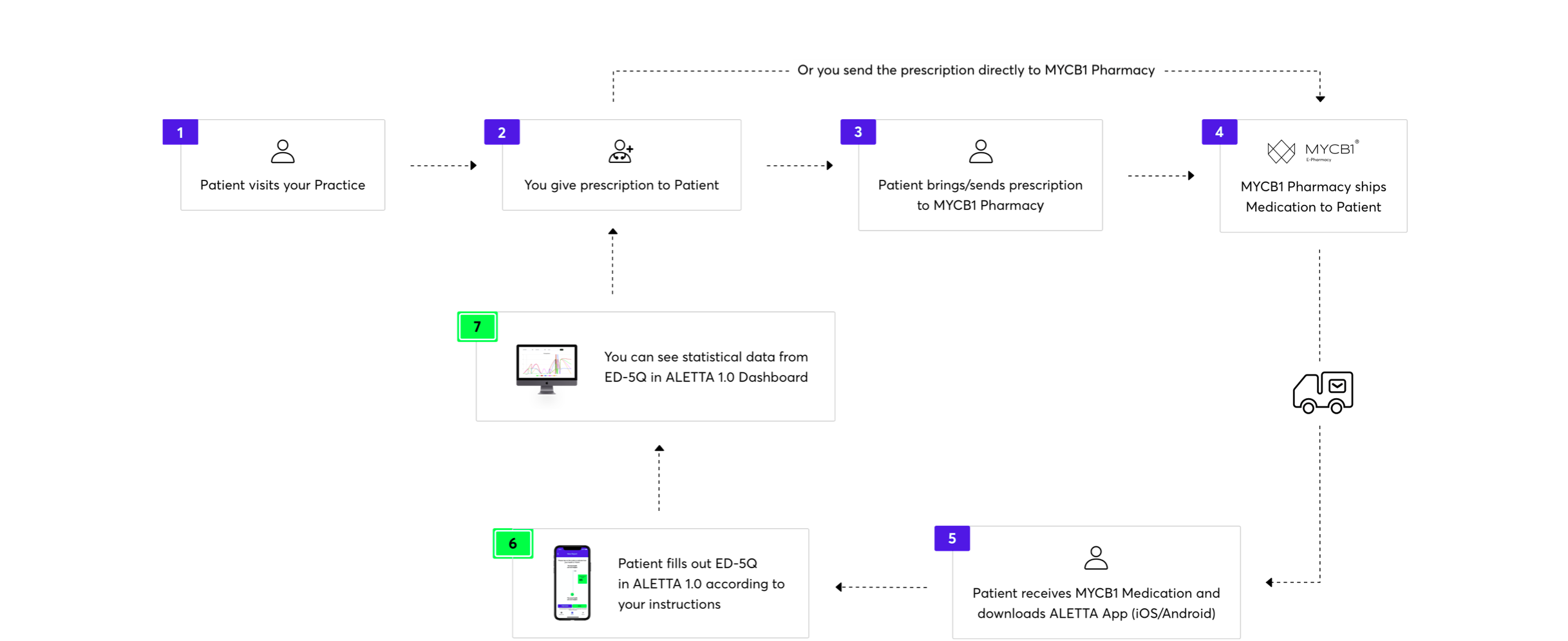
Patient journey.
Guidelines.
Active Product: CP for Neuropathic Pain.
A product is active when the EQ-5D psychometric properties for the specified condition have been systematically reviewed, whereby data collected on this product can be used in health economic models.

| Condition: | Neuropathic Pain |
| Product information: | Click here |
| Psychometric properties: | Click here |
Products portfolio.

| Chronic Pain |

| Therapy Resisten Glaucoma |

| Epilepsy |

| Spasticity |

| Chemotherapy nausea and vomiting |

| Pain in Cancer |

| Tourette syndrome |

| Pain in Arthritis |

| ADHD |

| Weight loss |

| Fibromyalgia |

| Rheumatism |
* Medicinal Cannabis cannot cure the conditions mentioned on this website, and the names on the boxes are products “codes” because of MYCB1 data-driven approach, and not drugs holding Marketing Authorization.
* “Cannabis can ease the symptoms of the disorders or reduce the side-effects of medicines. A healthcare professional decides, whether a patient can benefit from medicinal cannabis.” Source: Ministry of Health, Welfare and Sports Office of Medicinal Cannabis
Adherence.
MYCB1 is in a continuous innovation to optimise patient adherence to medication. Most of our patients need to use the medication 2 to 4 times a day, and the bottle and syringe for oral application must be at hand during daily life activities. In addition, the medication must be protected from UV light and abrupt temperature changes.
The custom designed Travel Kit by MYCB1 , is delivered for free to the patient with their first prescription; the thermal bag protects from UV light and temperature changes; and is user-friendly for the patient to carry and use the medication on the go.
Security.

ALETTA 1.0 is part of MYCB1 B.V. Apotheek, an ISO 27001:2017 and NEN 7510-1:2017 certified platform.
Scope of certification:
Protect the information used by the manufacturing of narcotic prescription products, internal communications, external communications with clients, the general public and collecting clinical data by a mobile app with the use of a Cloud Platform.
- Statement of Applicability available under request: Brandcompliance
- Click here to access original certifications


MYCB1 B.V. is partner of The Hague Security Delta (HSD)
The Dutch security cluster ‘The Hague Security Delta’ is a network of businesses, governments and knowledge institutions, that work together on knowledge development and innovation in security. More info
MYCB1 products are serialised and ALETTA 1.0 uses counterfeiting protection technology.
DISCLAIMER:
ALETTA 1.0 is using the validated questionnaire EQ-5D by EuroQol Research foundation. It is not a medical device, and has no medical applications.
What is ALETTA 1.0?
ALETTA 1.0 is a cloud base Real-World Data management platform built in with the validate questionnaire EQ-5D, a standardised instrument for generic health status. ALETTA is designed for self-completion and as such captures information directly from the patient using a mobile App available on the Android and Apple Store.
Use ALETTA 1.0 to assess Patient Quality of Life under MYCB1 cannabinoids treatment:
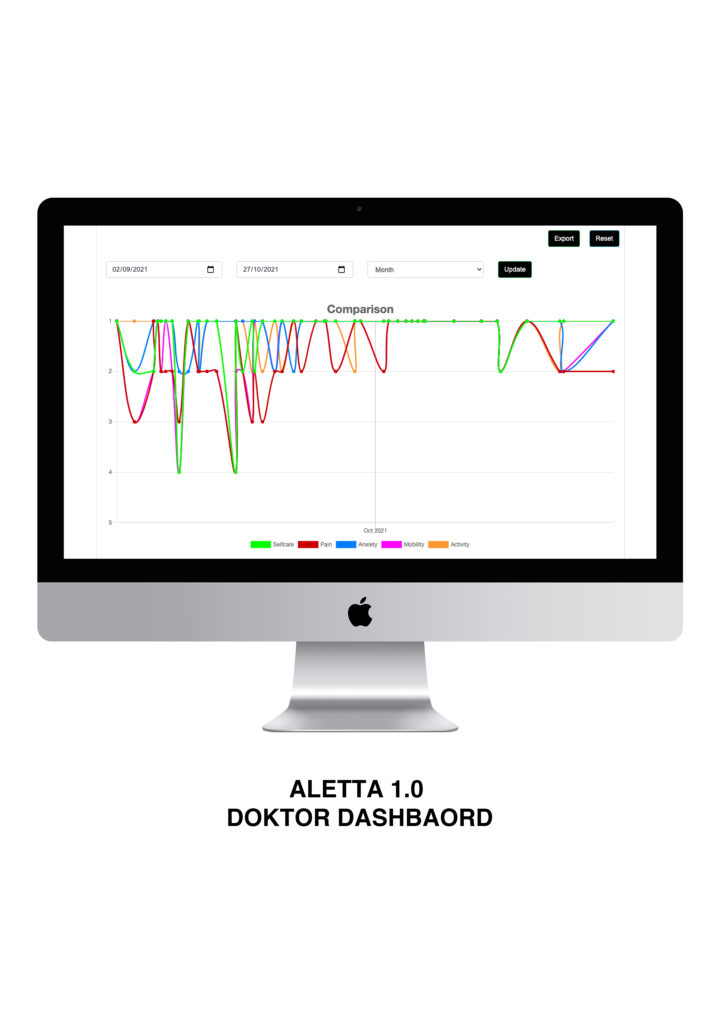
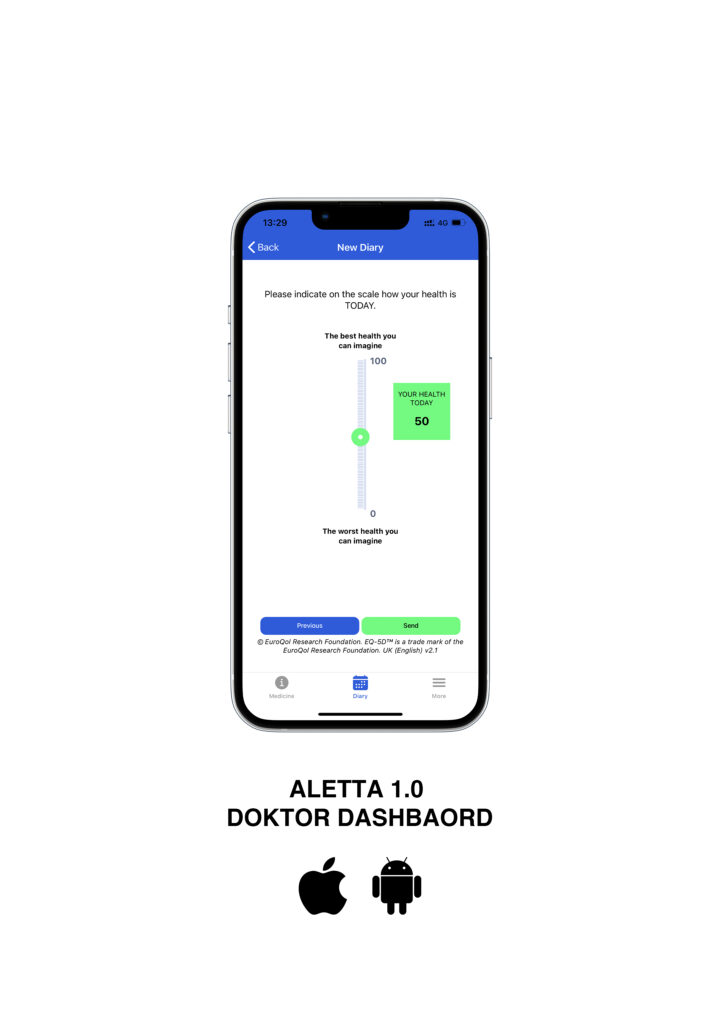
Patient journey.

Guidelines.
Active Product: CP for Neuropathic Pain.

| Condition: | Neuropathic Pain |
| Product information: | Click here |
| Psychometric properties: | Click here |
Products portfolio.

| Chronic Pain |

| Therapy Resisten Glaucoma |

| Epilepsy |

| Spasticity |

| Chemotherapy nausea and vomiting |

| Pain in Cancer |

| Tourette syndrome |

| Pain in Arthritis |

| ADHD |

| Weight loss |

| Fibromyalgia |

| Rheumatism |
* Medicinal Cannabis cannot cure the conditions mentioned on this website, and the names on the boxes are products “codes” because of MYCB1 data-driven approach, and not drugs holding Marketing Authorization.
* “Cannabis can ease the symptoms of the disorders or reduce the side-effects of medicines. A healthcare professional decides, whether a patient can benefit from medicinal cannabis.” Source: Ministry of Health, Welfare and Sports Office of Medicinal Cannabis
Adherence.
MYCB1 is in a continuous innovation to optimise patient adherence to medication. Most of our patients need to use the medication 2 to 4 times a day, and the bottle and syringe for oral application must be at hand during daily life activities. In addition, the medication must be protected from UV light and abrupt temperature changes.
The custom designed Travel Kit by MYCB1 , is delivered for free to the patient with their first prescription; the thermal bag protects from UV light and temperature changes; and is user-friendly for the patient to carry and use the medication on the go.
Security.

ALETTA 1.0 is part of MYCB1 B.V. Apotheek, an ISO 27001:2017 and NEN 7510-1:2017 certified platform.
Scope of certification:
Protect the information used by the manufacturing of narcotic prescription products, internal communications, external communications with clients, the general public and collecting clinical data by a mobile app with the use of a Cloud Platform.
- Statement of Applicability available under request: Brandcompliance
- Click here to access original certifications


MYCB1 B.V. is partner of The Hague Security Delta (HSD)
The Dutch security cluster ‘The Hague Security Delta’ is a network of businesses, governments and knowledge institutions, that work together on knowledge development and innovation in security. More info
MYCB1 products are serialised and ALETTA 1.0 uses counterfeiting protection technology.
